Marking Scheme: +1 for correct answers, 0 for incorrect answers, 0 for unattempted questions. Options cannot be deselected.
Exam Summary
0 of 15 Questions completed
Questions:
Information
You have already completed the exam before. Hence you can not start it again.
Exam is loading…
You must sign in or sign up to start the exam.
You must first complete the following:
Results
Results
0 of 15 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 mark(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 15
1. Question
1 Marks(s)The process with negative entropy change is
CorrectIncorrect -
Question 2 of 15
2. Question
1 Marks(s)An ideal gas undergoes isothermal compression from $$5 \mathrm{~m}^{3}$$ to $$1 \mathrm{~m}^{3}$$ against a constant external pressure of $$4 \mathrm{Nm}^{-2}$$. Heat released in this process is used to increase the temperature of 1 mole of Al . If molar heat capacity of Al is $$24 \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1}$$, the temperature of Al increases by
CorrectIncorrect -
Question 3 of 15
3. Question
1 Marks(s)Consider the reversible isothermal expansion of an ideal gas in a closed system at two different temperatures $$T_{1}$$ and $$T_{2}\left(T_{1}<T_{2}\right)$$. The correct graphical depiction of the dependence of work done (w) on the final volume $$(\mathrm{V})$$ is
CorrectIncorrect -
Question 4 of 15
4. Question
1 Marks(s)A process has $$\Delta H=200 \mathrm{Jmol}^{-1}$$ and $$\Delta S=40 \mathrm{JK}^{-1} \mathrm{~mol}^{-}$$. Out of the values given below, choose the minimum temperature above which the process will be spontaneous
CorrectIncorrect -
Question 5 of 15
5. Question
1 Marks(s)For the equilibrium, $$2 \mathrm{H}_{2} \mathrm{O} \leftrightarrow \mathrm{H}_{3} \mathrm{O}^{+}+\mathrm{OH}^{-}$$, the value of $$\Delta G^{0}$$ at 298 K is approximately
CorrectIncorrect -
Question 6 of 15
6. Question
1 Marks(s)The reaction, $$M g O(s)+C(s) \longrightarrow M g(s)+C O(g)$$, for which $$\Delta_{r} H^{0}=+491.1 \mathrm{~kJ} \mathrm{~mol}^{-1}$$ and $$\Delta_{r} S^{0}=$$ $$198.0 \mathrm{KK}^{-1} \mathrm{~mol}^{-1}$$, is not feasible at 298 K . Temperature above which reaction will be feasible is
CorrectIncorrect -
Question 7 of 15
7. Question
1 Marks(s)The standard reaction Gibbs energy for a chemical reaction at an absolute temperature T is given by $$\Delta_{r} G^{0}=A-B T$$ Where A and B are non-zero constants. Which of the following is TRUE about this reaction?
CorrectIncorrect -
Question 8 of 15
8. Question
1 Marks(s)The entropy change associated with the conversion of 1 kg of ice at 273 K to water vapours at 383 K is (Specific heat of water liquied and water vapour are $$4.2 \mathrm{~kJ}^{-1} \mathrm{~kg}^{-}$$and $$2.0 \mathrm{~kJ}^{-1} \mathrm{~kg}^{-}$$; heat of liquid fusion and vapourisation of water are $$334 \mathrm{~kJ}^{-1}$$ and $$2491 \mathrm{~kJ} \mathrm{~kg}^{-}$$, respectively). ( $$\log 273=2.436, \log$$ $$373=2.572, \log 383=2.583)$$
CorrectIncorrect -
Question 9 of 15
9. Question
1 Marks(s)Two blocks of the same metal having same mass and at temperature $$T_{1} a n d T_{2}$$, respectively, are brought in contact with each other and allowed to attain thermal equilibrium at constant pressure. The change in entropy, $$\Delta \mathrm{S}$$, for this process is
CorrectIncorrect -
Question 10 of 15
10. Question
1 Marks(s)For the chemical reaction $$X \leftrightarrow Y$$, the standard reaction Gibbs energy depends on temperature T (in K ) as
$$\Delta_{r} G^{0}\left(\right.$$ in kJ mol $$\left.^{-1}\right)=120-\frac{3}{8} T$$. The major component of the reaction mixture at T isCorrectIncorrect -
Question 11 of 15
11. Question
1 Marks(s)For a diatomic ideal gas in a closed system, which of the following plots does not correctly describe the relation between various thermodynamic quantities?
CorrectIncorrect -
Question 12 of 15
12. Question
1 Marks(s)Given,
i) $$\mathrm{C}($$ graphite $$)+\mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{CO}_{2}(\mathrm{~g}) ; \Delta_{r} \mathrm{H}^{\Theta}=x \mathrm{kJmol}^{-1}$$
ii) $$C($$ graphite $$)+\frac{1}{2} \mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{CO}(\mathrm{g}) ; \Delta_{r} \mathrm{H}^{\Theta}=\mathrm{ykJol}^{-1}$$
iii) $$\mathrm{CO}(g)+\frac{1}{2} \mathrm{O}_{2}(g) \rightarrow \mathrm{CO}_{2}(g) ; \Delta_{r} H^{\Theta}=\mathrm{zkJmol}^{-1}$$Based on the above thermochemical equations, find out which one of the following algebraic relationships is correct?
CorrectIncorrect -
Question 13 of 15
13. Question
1 Marks(s)Given i) $$2 \mathrm{Fe}_{2} \mathrm{O}_{2}(s) \rightarrow 4 \mathrm{Fe}(\mathrm{s})+3 \mathrm{O}_{2}(\mathrm{~g}) ; \Delta_{r} G^{0}=+1487.0 \mathrm{kJmol}^{-1}$$
ii) $$2 \mathrm{CO}(\mathrm{g})+\mathrm{O}_{2}(\mathrm{~g}) \rightarrow 2 \mathrm{CO}_{2}(\mathrm{~g}) ; \Delta_{r} G^{0}=-514.4 \mathrm{kJmol}^{-1}$$
free energy change, $$\Delta_{r} G^{0}$$ for the reaction $$2 \mathrm{Fe}_{2} \mathrm{O}_{3}(s)+6 \mathrm{CO}(\mathrm{g}) \rightarrow 4 \mathrm{Fe}(s)+6 \mathrm{CO}_{2}(g)$$ will beCorrectIncorrect -
Question 14 of 15
14. Question
1 Marks(s)$$\Delta_{r} G^{0}$$ at 500 K for substance ‘ S ” in liquid state and gaseous state are $$+100.7 \mathrm{kcal}^{-1} \mathrm{~mol}^{-1}$$ and +103 kcal $$\mathrm{mol}^{-1}$$, respectively. Vapour pressure of liquid ‘ S ‘ at 500 K is approximately equal to
CorrectIncorrect -
Question 15 of 15
15. Question
1 Marks(s)An ideal gas undergoes a cyclic process as shown in figure
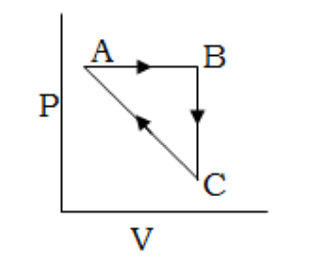
$$
\begin{aligned}
& \Delta U_{B C}=-5 \mathrm{kJmol}^{-1}, q_{A B}=2 \mathrm{kJmol}^{-1} \\
& W_{A B}=-5 \mathrm{kmol}^{-1}, W_{C A}=3 \mathrm{kmol}^{-1}
\end{aligned}
$$Heat absorbed by the system during process CA is
CorrectIncorrect