Marking Scheme: +1 for correct answers, 0 for incorrect answers, 0 for unattempted questions. Options cannot be deselected.
Exam Summary
0 of 15 Questions completed
Questions:
Information
You have already completed the exam before. Hence you can not start it again.
Exam is loading…
You must sign in or sign up to start the exam.
You must first complete the following:
Results
Results
0 of 15 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 mark(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 15
1. Question
1 Marks(s)The principle involved in the classification of basic radicals is/are
CorrectIncorrect -
Question 2 of 15
2. Question
1 Marks(s)$$\mathrm{Al}^{3+}, \mathrm{Fe}^{3+}$$ and $$\mathrm{Cr}^{3+}$$ are grouped together for qualitative analysis as their
CorrectIncorrect -
Question 3 of 15
3. Question
1 Marks(s)Addition of conc. HCl to saturated $$\mathrm{BaCl}_{2}$$ solution precipitates $$\mathrm{BaCl}_{2}$$ because
CorrectIncorrect -
Question 4 of 15
4. Question
1 Marks(s)Colourless salt
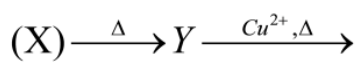 coloured bead, X can beCorrectIncorrect
coloured bead, X can beCorrectIncorrect -
Question 5 of 15
5. Question
1 Marks(s)Nitrate salt forms a brown ring treated with $$\mathrm{FeSO}_{4}(\mathrm{aq})$$ followed by a few drops of concentrated $$\mathrm{H}_{2} \mathrm{SO}_{4}$$. This test invoves
CorrectIncorrect -
Question 6 of 15
6. Question
1 Marks(s)In III group of basic radical analysis, $$\mathrm{NH}_{4} \mathrm{Cl}$$ is added before group reagent $$\mathrm{NH}_{4} \mathrm{OH}$$. This is due to
CorrectIncorrect -
Question 7 of 15
7. Question
1 Marks(s)$$\quad \mathrm{X}$$ is a colorless salt in cold but becomes yellow on heating. It is soluble in NaOH as well as in dilute HCl . X can be
CorrectIncorrect -
Question 8 of 15
8. Question
1 Marks(s)$$\quad \mathrm{H}_{2} \mathrm{~S}$$ gas is passed into test tubes A and B containing aqueous solution of
A. $${ZnCl}_{2}$$
B. $$\mathrm{Zn}\left(\mathrm{CH}_{3} \mathrm{COO}\right)_{2}$$The true statement about $$A$$ and $$B$$ is
CorrectIncorrect -
Question 9 of 15
9. Question
1 Marks(s)Chloride salt gives colourless fumes with conc. $$\mathrm{H}_{2} \mathrm{SO}_{4}$$ while iodide salt gives violet fumes in the same test. This is because
CorrectIncorrect -
Question 10 of 15
10. Question
1 Marks(s)A salt releases a colourless pungent gas ‘ $$X$$ ‘ when treated with dilute $$\mathrm{H}_{2} \mathrm{SO}_{4}$$. The released gas turns $$\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-} / \mathrm{H}^{+}$$green. The green colour is due to the formation of ‘ $$Y^{\prime}$$. ‘ $$X$$ ‘ is used in the manufacture of $$H_{2} \mathrm{SO}_{4}$$ by contact process. $$X$$ and $$Y$$ are respectively
CorrectIncorrect -
Question 11 of 15
11. Question
1 Marks(s)Mixture ” $$A$$ ” is an aqueous solution of two colourless substances $$X$$ and $$Y$$. On passing $${C l_{2}}(\mathrm{~g})$$ through the solution, a deep brown color is developed, while treatment of the solution with $${\mathrm{BaCl}_{2}}$$ or NaOH result in the formation of a white ppt. $$X$$ and $$Y$$ may be
CorrectIncorrect -
Question 12 of 15
12. Question
1 Marks(s)Through aqueous solution of an iodide salt when $${\mathrm{Cl}_{2}}$$ gas is passed followed by the addition of $$\mathrm{CHCl}_{3}$$, the color of organic layer will become
CorrectIncorrect -
Question 13 of 15
13. Question
1 Marks(s)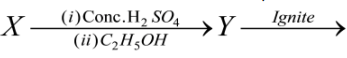
The volatile compound Y burning with green flame has the formula
CorrectIncorrect -
Question 14 of 15
14. Question
1 Marks(s)State whether the following statements are true or false and choose the correct option.
i. Brown ring test is a characteristic test for both $$\mathrm{NO}_{2}^{-}$$and $${NO}_{3}^{-}$$.
ii. A brown gas is evolved when either $${NO}_{2}^{-}$$or $$\mathrm{NO}_{3}^{-}$$is treated with concentrated solution of sulphuric acid.
iii. A nitrate salt gives $${NH}_{3}$$ when treated with alkali in the presence of copper metal.CorrectIncorrect -
Question 15 of 15
15. Question
1 Marks(s)Some facts related to compound $$X$$ are as follows:
i. It occupies 0.35 L per g at NTP
ii. It decolorizes acidified permanganate solution.
iii. Its aqueous solution produces white turbidity with $$\mathrm{H}_{2} \mathrm{~S}$$.Compound X may be
CorrectIncorrect