Marking Scheme: +1 for correct answers, 0 for incorrect answers, 0 for unattempted questions. Options cannot be deselected.
Exam Summary
0 of 15 Questions completed
Questions:
Information
You have already completed the exam before. Hence you can not start it again.
Exam is loading…
You must sign in or sign up to start the exam.
You must first complete the following:
Results
Results
0 of 15 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 mark(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 15
1. Question
1 Marks(s)The pKa values of the acids A to D are found to be $$4.19,3.41,4.46$$ and 4.76 . the acid having pKa of 3.41 is
CorrectIncorrect -
Question 2 of 15
2. Question
1 Marks(s)Which of the following phenols is most soluble in aqueous sodium bicarbonate?
CorrectIncorrect -
Question 3 of 15
3. Question
1 Marks(s)The order of acidities of the H -atoms underlined in the following compounds is in the order
i)$$\mathrm{Ph}-\underline{\mathrm{CH}_{2}}-\mathrm{CH}_{3}$$
ii)$$\mathrm{Ph}-\mathrm{C} \equiv \underline{\mathrm{CH}}$$
iii)$$\mathrm{Ph}-\mathrm{HC}=\underline{\mathrm{CH}_{2}}$$
iv)
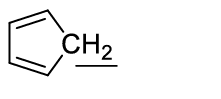 CorrectIncorrect
CorrectIncorrect -
Question 4 of 15
4. Question
1 Marks(s)The correct decreasing order for acid strength is?
CorrectIncorrect -
Question 5 of 15
5. Question
1 Marks(s)Which amongst the following is the strongest acid?
CorrectIncorrect -
Question 6 of 15
6. Question
1 Marks(s)The increasing order of the pKa values of the following compounds is?
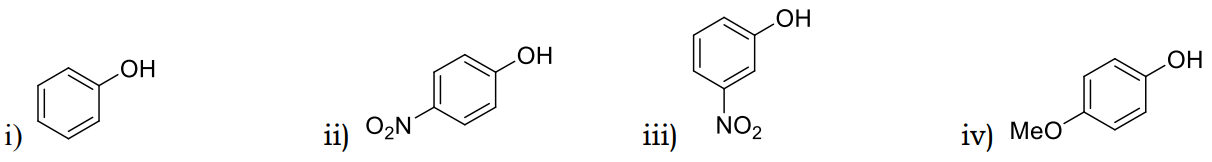 CorrectIncorrect
CorrectIncorrect -
Question 7 of 15
7. Question
1 Marks(s)The correct order for acid strength of compounds $$\mathrm{CH} \equiv \mathrm{CH}, \mathrm{CH}_{3}-\mathrm{C} \equiv \mathrm{CH}$$ and $$\mathrm{CH}_{2}=\mathrm{CH}_{2}$$ is as follows
CorrectIncorrect -
Question 8 of 15
8. Question
1 Marks(s)The alcohol which is having the highest Ka value
CorrectIncorrect -
Question 9 of 15
9. Question
1 Marks(s)Indicate the correct acidity (first ionization) in the following carboxylic acid
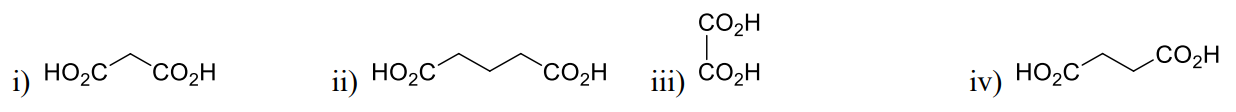 CorrectIncorrect
CorrectIncorrect -
Question 10 of 15
10. Question
1 Marks(s)the most acidic species among the following is
CorrectIncorrect -
Question 11 of 15
11. Question
1 Marks(s)Which of the following statements is true with respect to the following pair of compounds?
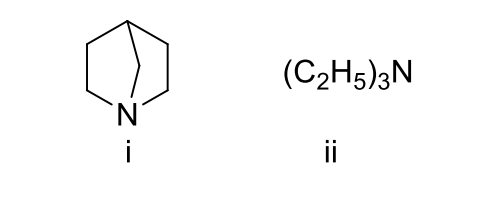 CorrectIncorrect
CorrectIncorrect -
Question 12 of 15
12. Question
1 Marks(s)Oxygen atom of the carbonyl group is most basic in
CorrectIncorrect -
Question 13 of 15
13. Question
1 Marks(s)The relative basic strengths of $$\mathrm{NH}_{3}, \mathrm{CH}_{3} \mathrm{NH}_{2}$$, and $$\mathrm{NF}_{3}$$ are in the order
CorrectIncorrect -
Question 14 of 15
14. Question
1 Marks(s)The order of acidity of the given series of the compounds is?
i) HCOOH
ii) $$\mathrm{CH}_{3} \mathrm{COOH}$$
iii) PhCOOH
CorrectIncorrect -
Question 15 of 15
15. Question
1 Marks(s)The compound that does not liberate $$\mathrm{CO}_{2}$$ on treatment with aqueous sodium bicarbonate solution is
CorrectIncorrect