Marking Scheme: +1 for correct answers, 0 for incorrect answers, 0 for unattempted questions Options cannot be deselected.
Exam Summary
0 of 30 Questions completed
Questions:
Information
You have already completed the exam before. Hence you can not start it again.
Exam is loading…
You must sign in or sign up to start the exam.
You must first complete the following:
Results
Results
0 of 30 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 mark(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 30
1. Question
1 Marks(s)Two substances $$A$$ and $$B$$ of equal mass $$m$$ are heated at uniform rate of $$6 \mathrm{cal}^{-1}$$ under similar conditions. A graph between temperature and time is shown in figure. Ratio of heat absorbed $$H_{A} / H_{B}$$ by them for complete fusion is
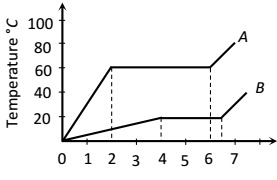 CorrectIncorrect
CorrectIncorrect -
Question 2 of 30
2. Question
1 Marks(s)Out of the following, in which vessel will the temperature of the solution be higher after the salt is completely dissolved.
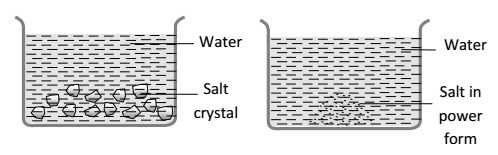 CorrectIncorrect
CorrectIncorrect -
Question 3 of 30
3. Question
1 Marks(s)Fire is extinguished more effectively by
CorrectIncorrect -
Question 4 of 30
4. Question
1 Marks(s)An ideal thermometer should have
CorrectIncorrect -
Question 5 of 30
5. Question
1 Marks(s)A steel meter scale is to be ruled so that millimeter intervals are accurate within about $$5 \times 10^{-5}$$ mm at a certain temperature. The maximum temperature variation allowable during the ruling is (Coefficient of linear expansion of steel $$=10 \times 10^{-6} \mathrm{~K}^{-1}$$ )
CorrectIncorrect -
Question 6 of 30
6. Question
1 Marks(s)During illness an 80 kg man ran a fever of $$102.2^{\circ} \mathrm{F}$$ instead of normal body temperature of $$98.6^{\circ} \mathrm{F}$$. Assuming that human body is mostly water, how much heat is required to raise his temperature by that amount
CorrectIncorrect -
Question 7 of 30
7. Question
1 Marks(s)Two holes of unequal diameters $$d_{1}$$ and $$d_{2}\left(d_{1}>d_{2}\right)$$ are cut in a metal sheet. If the sheet is heated
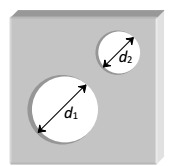 CorrectIncorrect
CorrectIncorrect -
Question 8 of 30
8. Question
1 Marks(s)If earth suddenly stops rotating about its own axis, the increase in it’s temperature will be
CorrectIncorrect -
Question 9 of 30
9. Question
1 Marks(s)Latent heat of ice is $$80 \mathrm{cal} / \mathrm{gm}$$. A man melts 60 g of ice by chewing in 1 minute. His power is
CorrectIncorrect -
Question 10 of 30
10. Question
1 Marks(s)A faulty thermometer has its lower fixed point marked as $$-10^{\circ} \mathrm{C}$$ and upper fixed point marked as $$110^{\circ}$$ and upper fixed point marked as $$110^{\circ}$$. If the temperature of the body shown in this scale is $$62^{\circ}$$, the temperature shown on the Celsius scale is
CorrectIncorrect -
Question 11 of 30
11. Question
1 Marks(s)If there are no heat losses, the heat released by the condensation of $$x \mathrm{gm}$$ of steam at $$100^{\circ} \mathrm{C}$$ into water at $$100^{\circ} \mathrm{C}$$ can be used to convert $$y \mathrm{gm}$$ of ice at $$0^{\circ} \mathrm{C}$$ into water at $$100^{\circ} \mathrm{C}$$. Then the ratio $$y: x$$ is nearly
CorrectIncorrect -
Question 12 of 30
12. Question
1 Marks(s)The figure shows a glass tube (linear co-efficient of expansion is $$\alpha$$ ) completely filled with a liquid of volume expansion co-efficient $$\gamma$$. On heating length of the liquid column does not change. Choose the correct relation between $$\gamma$$ and $$\alpha$$
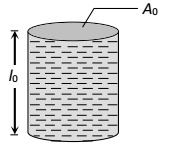 CorrectIncorrect
CorrectIncorrect -
Question 13 of 30
13. Question
1 Marks(s)Water falls from a height 500 m . What is the rise in temperature of water at bottom if whole energy remains in the water
CorrectIncorrect -
Question 14 of 30
14. Question
1 Marks(s)A steel ball of mass 0.1 kg falls freely from a height of 10 m and bounces to a height of 5.4 m from the ground. If the dissipated energy in this process is absorbed by the ball, the rise in its temperature is
(Specific heat of steel $$=460$$ Joule $$-\mathrm{kg}^{-10} \mathrm{C}^{-1}, g=10 \mathrm{~ms}^{-2}$$ )CorrectIncorrect -
Question 15 of 30
15. Question
1 Marks(s)1 gm of ice at $$0^{\circ} \mathrm{C}$$ is mixed with 1 gm of water at $$100^{\circ} \mathrm{C}$$ the resulting temperature will be
CorrectIncorrect -
Question 16 of 30
16. Question
1 Marks(s)Two substances $$A$$ and $$B$$ of equal mass $$m$$ are heated at uniform rate of $$6 \mathrm{cal}^{-1}$$ under similar conditions. A graph between temperature and time is shown in figure. Ratio of heat absorbed $$H_{A} / H_{B}$$ by them for complete fusion is
 CorrectIncorrect
CorrectIncorrect -
Question 17 of 30
17. Question
1 Marks(s)Out of the following, in which vessel will the temperature of the solution be higher after the salt is completely dissolved.
 CorrectIncorrect
CorrectIncorrect -
Question 18 of 30
18. Question
1 Marks(s)Fire is extinguished more effectively by
CorrectIncorrect -
Question 19 of 30
19. Question
1 Marks(s)An ideal thermometer should have
CorrectIncorrect -
Question 20 of 30
20. Question
1 Marks(s)A steel meter scale is to be ruled so that millimeter intervals are accurate within about $$5 \times 10^{-5}$$ mm at a certain temperature. The maximum temperature variation allowable during the ruling is (Coefficient of linear expansion of steel $$=10 \times 10^{-6} \mathrm{~K}^{-1}$$ )
CorrectIncorrect -
Question 21 of 30
21. Question
1 Marks(s)During illness an 80 kg man ran a fever of $$102.2^{\circ} \mathrm{F}$$ instead of normal body temperature of $$98.6^{\circ} \mathrm{F}$$. Assuming that human body is mostly water, how much heat is required to raise his temperature by that amount
CorrectIncorrect -
Question 22 of 30
22. Question
1 Marks(s)Two holes of unequal diameters $$d_{1}$$ and $$d_{2}\left(d_{1}>d_{2}\right)$$ are cut in a metal sheet. If the sheet is heated
 CorrectIncorrect
CorrectIncorrect -
Question 23 of 30
23. Question
1 Marks(s)If earth suddenly stops rotating about its own axis, the increase in it’s temperature will be
CorrectIncorrect -
Question 24 of 30
24. Question
1 Marks(s)Latent heat of ice is $$80 \mathrm{cal} / \mathrm{gm}$$. A man melts 60 g of ice by chewing in 1 minute. His power is
CorrectIncorrect -
Question 25 of 30
25. Question
1 Marks(s)A faulty thermometer has its lower fixed point marked as $$-10^{\circ} \mathrm{C}$$ and upper fixed point marked as $$110^{\circ}$$ and upper fixed point marked as $$110^{\circ}$$. If the temperature of the body shown in this scale is $$62^{\circ}$$, the temperature shown on the Celsius scale is
CorrectIncorrect -
Question 26 of 30
26. Question
1 Marks(s)If there are no heat losses, the heat released by the condensation of $$x \mathrm{gm}$$ of steam at $$100^{\circ} \mathrm{C}$$ into water at $$100^{\circ} \mathrm{C}$$ can be used to convert $$y \mathrm{gm}$$ of ice at $$0^{\circ} \mathrm{C}$$ into water at $$100^{\circ} \mathrm{C}$$. Then the ratio $$y: x$$ is nearly
CorrectIncorrect -
Question 27 of 30
27. Question
1 Marks(s)The figure shows a glass tube (linear co-efficient of expansion is $$\alpha$$ ) completely filled with a liquid of volume expansion co-efficient $$\gamma$$. On heating length of the liquid column does not change. Choose the correct relation between $$\gamma$$ and $$\alpha$$
 CorrectIncorrect
CorrectIncorrect -
Question 28 of 30
28. Question
1 Marks(s)Water falls from a height 500 m . What is the rise in temperature of water at bottom if whole energy remains in the water
CorrectIncorrect -
Question 29 of 30
29. Question
1 Marks(s)A steel ball of mass 0.1 kg falls freely from a height of 10 m and bounces to a height of 5.4 m from the ground. If the dissipated energy in this process is absorbed by the ball, the rise in its temperature is
(Specific heat of steel $$=460$$ Joule $$-\mathrm{kg}^{-10} \mathrm{C}^{-1}, g=10 \mathrm{~ms}^{-2}$$ )CorrectIncorrect -
Question 30 of 30
30. Question
1 Marks(s)1 gm of ice at $$0^{\circ} \mathrm{C}$$ is mixed with 1 gm of water at $$100^{\circ} \mathrm{C}$$ the resulting temperature will be
CorrectIncorrect