Marking Scheme: +1 for correct answers, 0 for incorrect answers, 0 for unattempted questions. Options cannot be deselected.
Exam Summary
0 of 15 Questions completed
Questions:
Information
You have already completed the exam before. Hence you can not start it again.
Exam is loading…
You must sign in or sign up to start the exam.
You must first complete the following:
Results
Results
0 of 15 Questions answered correctly
Your time:
Time has elapsed
You have reached 0 of 0 mark(s), (0)
Earned Point(s): 0 of 0, (0)
0 Essay(s) Pending (Possible Point(s): 0)
| Average score |
|
| Your score |
|
Categories
- Not categorized 0%
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- Current
- Review
- Answered
- Correct
- Incorrect
-
Question 1 of 15
1. Question
1 Marks(s)For which of the following reactions, $$\Delta H$$ is equal to $$\Delta U$$ ?
CorrectIncorrect -
Question 2 of 15
2. Question
1 Marks(s)For which of the following processes. $$\Delta \mathrm{S}$$ is negative?
CorrectIncorrect -
Question 3 of 15
3. Question
1 Marks(s)At 320 K , a gas $$A_{2}$$ is $$20 \%$$ dissociated to $$\mathrm{A}(\mathrm{g})$$. The standard free energy change at 320 K and 1 atm in J $$\mathrm{mol}^{-1}$$ is approximately
( $$\left.R=8.314 \mathrm{JK}^{-1} \mathrm{~mol}^{-1} ; \ln 2=0.693 ; \ln 3=1.098\right)$$CorrectIncorrect -
Question 4 of 15
4. Question
1 Marks(s)For a reaction, $$\mathrm{A}(\mathrm{g}) \rightarrow \mathrm{Al}) ; \Delta \mathrm{H}=-3 \mathrm{RT}$$. The correct statement for the reaction is
CorrectIncorrect -
Question 5 of 15
5. Question
1 Marks(s)The enthalpy change on freezing of 1 mol of water at $$5^{\circ} \mathrm{C}$$ to ice at $$-5^{\circ} \mathrm{C}$$ is:
(Given $$\Delta_{f u s} H=6 \mathrm{kmol}^{-1}$$ at $$0^{0} \mathrm{C}, C_{p}\left(H_{2} \mathrm{O}, l\right)=75.3 \mathrm{~J} \mathrm{~mol}^{-1} \mathrm{~K}^{-1} \mathrm{C}_{p}\left(\mathrm{H}_{2} \mathrm{O}, \mathrm{s}\right)=36.8 \mathrm{Jmol}^{-1} \mathrm{~K}^{-1}$$ )CorrectIncorrect -
Question 6 of 15
6. Question
1 Marks(s)A mixture of 2 mole of carbon monoxide gas and one mole of dioxygen gas is enclosed in a vessel and is ignited to convert carbon monoxide into carbon dioxide. If the enthalpy change is $$\Delta \mathrm{H}$$ and internal energy change is $$\Delta U$$, then for the above process –
CorrectIncorrect -
Question 7 of 15
7. Question
1 Marks(s)The enthalpy of neutralization of $$\mathrm{NH}_{4} \mathrm{OH}$$ and $$\mathrm{CH}_{3} \mathrm{COOH}$$ is $$-10.5 \mathrm{kcal} / \mathrm{mole}$$ and enthalpy of neutralization of strong base and $$\mathrm{CH}_{3} \mathrm{COOH}$$ is $$-12.5 \mathrm{kcal} / \mathrm{mole}$$. Calculate the enthalpy of bond dissociation of base $$\mathrm{NH}_{4} \mathrm{OH}-$$
CorrectIncorrect -
Question 8 of 15
8. Question
1 Marks(s)Given that
$$\mathrm{CO}(\mathrm{g})+\mathrm{O}_{2}(\mathrm{~g}) \rightarrow \mathrm{CO}_{2}(\mathrm{~g}) ; \Delta \mathrm{H}^{\mathrm{o}}=-\mathrm{x} \mathrm{kJ}$$
which of the following is correct –CorrectIncorrect -
Question 9 of 15
9. Question
1 Marks(s)For the reaction,
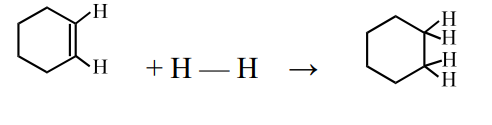
bond energies are given as under –
(i) $$\mathrm{C}-\mathrm{C}, 346 \mathrm{~kJ} / \mathrm{mol}$$
(ii) $$\mathrm{C}-\mathrm{H}, 413 \mathrm{~kJ} / \mathrm{mol}$$
(iii) $$\mathrm{H}-\mathrm{H}, 437 \mathrm{~kJ} / \mathrm{mol}$$ and
(iv) $$\mathrm{C}=\mathrm{C}, 611 \mathrm{~kJ} / \mathrm{mol}$$What will be the value of $$\Delta \mathrm{H} 25^{\circ} \mathrm{C}$$ for the above reaction ?
CorrectIncorrect -
Question 10 of 15
10. Question
1 Marks(s)Latent heat of vaporisation of water is $$540 \mathrm{cal} \mathrm{g}^{-1}$$. The entropy change during the evaporation of 1 mole of water at $$100^{\circ} \mathrm{C}$$ is –
CorrectIncorrect -
Question 11 of 15
11. Question
1 Marks(s)The solubility product of AgCl is $$1.6 \times 10^{-10}$$ and $$\log$$ ksp is -9.80 . The value of $$\Delta\mathrm{G}^{\circ}$$ for the process, $$\mathrm{AgCl}(\mathrm{s})+\mathrm{aq} \rightleftharpoons \mathrm{Ag}^{+}(\mathrm{aq})+\mathrm{Cl}^{-}(\mathrm{aq})$$ is –
CorrectIncorrect -
Question 12 of 15
12. Question
1 Marks(s)Which of the following process involves decrease in the entropy of system –
CorrectIncorrect -
Question 13 of 15
13. Question
1 Marks(s)The bond enthalpies of $$\mathrm{H}_{2}, \mathrm{X}_{2}$$ and HX are in the ratio of $$2: 1: 2$$. if the enthalpy for formation of HX is $$-50 \mathrm{~kJ} \mathrm{~mol}^{-1}$$, the bond enthalpy if $$\mathrm{H}_{2}$$ is –
CorrectIncorrect -
Question 14 of 15
14. Question
1 Marks(s)$$\Delta \mathrm{S}^{\circ}$$ and $$\Delta \mathrm{H}^{\circ}$$ for combustion of methane are $$186 \mathrm{JK}^{-1}$$ and $$-74.8 \mathrm{~kJ} \mathrm{~mol}^{-1}$$ respectively. The value of $$\Delta \mathrm{U}^{\circ}$$ for the process would be –
CorrectIncorrect -
Question 15 of 15
15. Question
1 Marks(s)The standard free energy change $$\Delta G^{\circ}$$ is related to equilibrium constant, $$K_{p}$$ as –
CorrectIncorrect